What’s new in acne: Skin and gut microbiome modulation in acne
Brigitte Dréno (France)
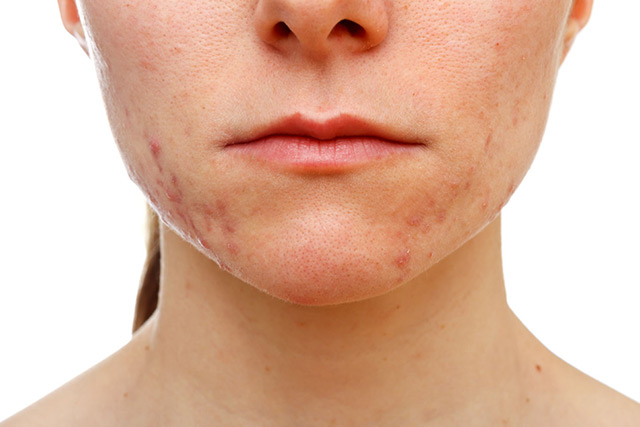
Acne vulgaris is a chronic inflammatory skin disease with a complex pathogenesis. The primary pathophysiologic factors in acne have been thought to be: altered sebum production, excess keratinization, and proliferation of a commensal bacteria, Cutibacterium acnes. However, the role of C. acnes has been unclear, since virtually all adults have C. acnes on their skin, and yet not all of them develop acne.
In recent years, understanding of the role of C. acnes has expanded. It is still acknowledged to have an important place in acne pathogenesis, but evidence suggests that an imbalance of individual C. acnes phylotypes with a predominance of the phylotype 1AI acts as an acne trigger. In addition, it is now believed that Staphylococcus epidermidis is also a factor in acne development. Together, C. acnes and S. epidermidis maintain and regulate the homeostasis of the skin microbiota. Antibiotics, which have long been a staple of acne therapy, induce cutaneous dysbiosis. In addition, very recently interactions between the gut microbiome and the sebaceous gland have been identified, which could be one of the ways by which diet and mainly sugar could play a role in acne. These findings, together with the long-standing public health edict to spare antibiotic use, when possible, highlight the need for a change in acne management strategies, opening the door to probiotics (substances that influence the growth of microbiome) and prebiotics (products secreted by the microbiome).


What’s new in acne: Spectrum of severe forms of acne
Clio Dessinioti (Greece)
In this presentation, we’ll delve into the different types of severe acne, such as nodulocystic acne, acne conglobata (a severe form of acne vulgaris), as well as severe acne variants such as acne fulminans (acne maligna), and severe acne manifesting as part of syndromes, such as SAPHO (synovitis, acne pustulosis, hyperostosis and osteitis) syndrome. We’ll discuss the clinical characteristics of these conditions, how they are diagnosed, and explore different treatment approaches.
In recent years, understanding of the role of C. acnes has expanded. It is still acknowledged to have an important place in acne pathogenesis, but evidence suggests that an imbalance of individual C. acnes phylotypes with a predominance of the phylotype 1AI acts as an acne trigger. In addition, it is now believed that Staphylococcus epidermidis is also a factor in acne development. Together, C. acnes and S. epidermidis maintain and regulate the homeostasis of the skin microbiota. Antibiotics, which have long been a staple of acne therapy, induce cutaneous dysbiosis. In addition, very recently interactions between the gut microbiome and the sebaceous gland have been identified, which could be one of the ways by which diet and mainly sugar could play a role in acne. These findings, together with the long-standing public health edict to spare antibiotic use, when possible, highlight the need for a change in acne management strategies, opening the door to probiotics (substances that influence the growth of microbiome) and prebiotics (products secreted by the microbiome).


What’s new in hidradenitis suppurativa: Comorbidities
John Ingram (United Kingdom)
Hidradenitis suppurativa (HS) comorbidities are well-established in European populations. There are associations between HS and other cardiovascular (CV) disease risk factors, including type 2 diabetes and metabolic syndrome. People with HS have double the risk of death from CV disease compared to those without HS and 1.5 times the risk compared to psoriasis patients. Depression and anxiety are associated with HS and completed suicide rates in those with HS are more than double the rates in controls. Associations exist between HS and other chronic inflammatory conditions, particularly inflammatory bowel disease and inflammatory arthritis. Case-control studies demonstrate associations with pilonidal sinus, polycystic ovary syndrome, Down syndrome, obstructive sleep apnoea and pyoderma gangrenosum.